15.2d Ex4 MJ17 P42 Q2 Specific Heat Capacity for Gas | A2 Ideal Gas | Cambridge A Level 9702 Physics
Автор: ETphysics
Загружено: 2020-11-27
Просмотров: 12840
Описание:
Example 4 - 9702/42/M/J/17: The pressure p and volume V of an ideal gas are related to the density ρ of the gas by the expression P = 3/2ρ〈c^2〉.
(ii) Use the expression to show that the mean kinetic energy EK of a gas molecule is given by EK = 3/2kT where k is the Boltzmann constant and T is the thermodynamic temperature.
#9702s17p42 #A2kineticTheoryP4 #Lv3
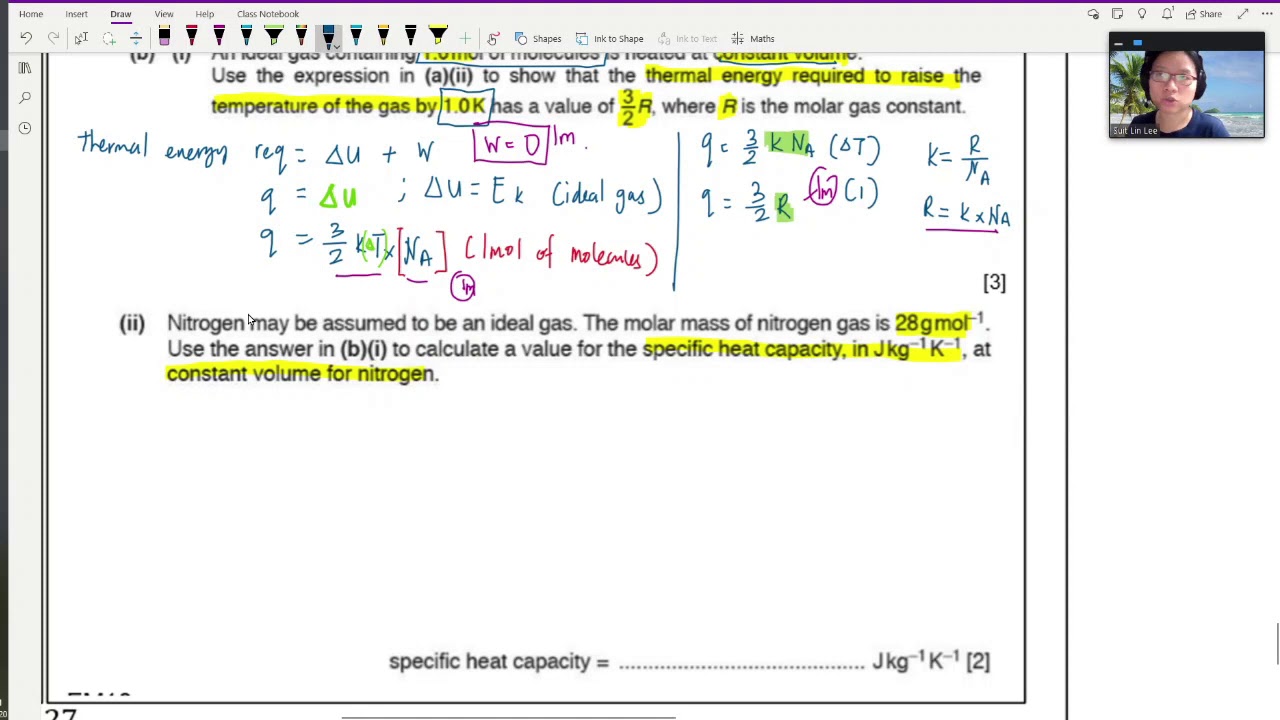
(b) (i) An ideal gas containing 1.0 mol of molecules is heated at constant volume. Use the expression in (a)(ii) to show that the thermal energy required to raise the temperature of the gas by 1.0 K has a value of 23R, where R is the molar gas constant
(ii) Nitrogen may be assumed to be an ideal gas. The molar mass of nitrogen gas is 28 g mol–1.Use the answer in (b)(i) to calculate a value for the specific heat capacity, in J kg–1 K–1, at constant volume for nitrogen
#A2IdealGas and #A2Thermodynamics Playlist:
• Ch15-16 Ideal Gas & Thermodynamics | CAIE ...
Instagram Pocket Guide ( / etphysics )
More Resources (https://9702-physics.blogspot.com/)
Support us! ( / etphysics )
Tuition/Tutoring inquiries:
Email: [email protected]
Whatsapp: https://wa.me/60126679171
#CambridgeALevelPhysics
Повторяем попытку...
Доступные форматы для скачивания:
Скачать видео
-
Информация по загрузке:














![ARMIA PUTINA DRUGĄ ARMIĄ ŚWIATA? PRAWDZIWA SIŁA ROSJI [WOLSKI I BOJKE]](https://imager.clipsaver.ru/-0hLR4R3gvk/max.jpg)




