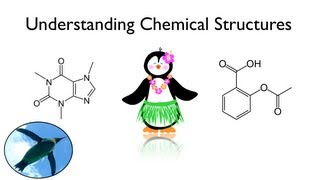
Esters vs. Carboxylic Acids: Structures, Differences, and Examples
Автор: Wayne Breslyn (Dr. B.)
Загружено: 2025-03-15
Просмотров: 2315
Описание:
In this video, we’ll explore the differences between esters and carboxylic acids—two essential functional groups in organic chemistry. Starting with their general structures, we’ll clarify what sets esters and carboxylic acids apart, then move on to specific examples and practice exercises.
Ester: Esters feature a carbonyl group (C=O) bonded to an oxygen atom, which is also connected to another carbon group (R–C=O–O–R). Esters are commonly found in fragrances and flavors due to their pleasant aromas.
Carboxylic Acid: Carboxylic acids contain a carbonyl group (C=O) bonded to an -OH group, creating the -COOH structure. Known for their acidic properties, carboxylic acids are commonly found in biological and industrial compounds.
This video offers a clear, step-by-step comparison with examples and exercises to reinforce your understanding of esters and carboxylic acids.
Повторяем попытку...

Доступные форматы для скачивания:
Скачать видео
-
Информация по загрузке: