Common Technical Documents - Drug Regulatory Affairs
Автор: Cliniminds India
Загружено: 2025-06-12
Просмотров: 256
Описание:
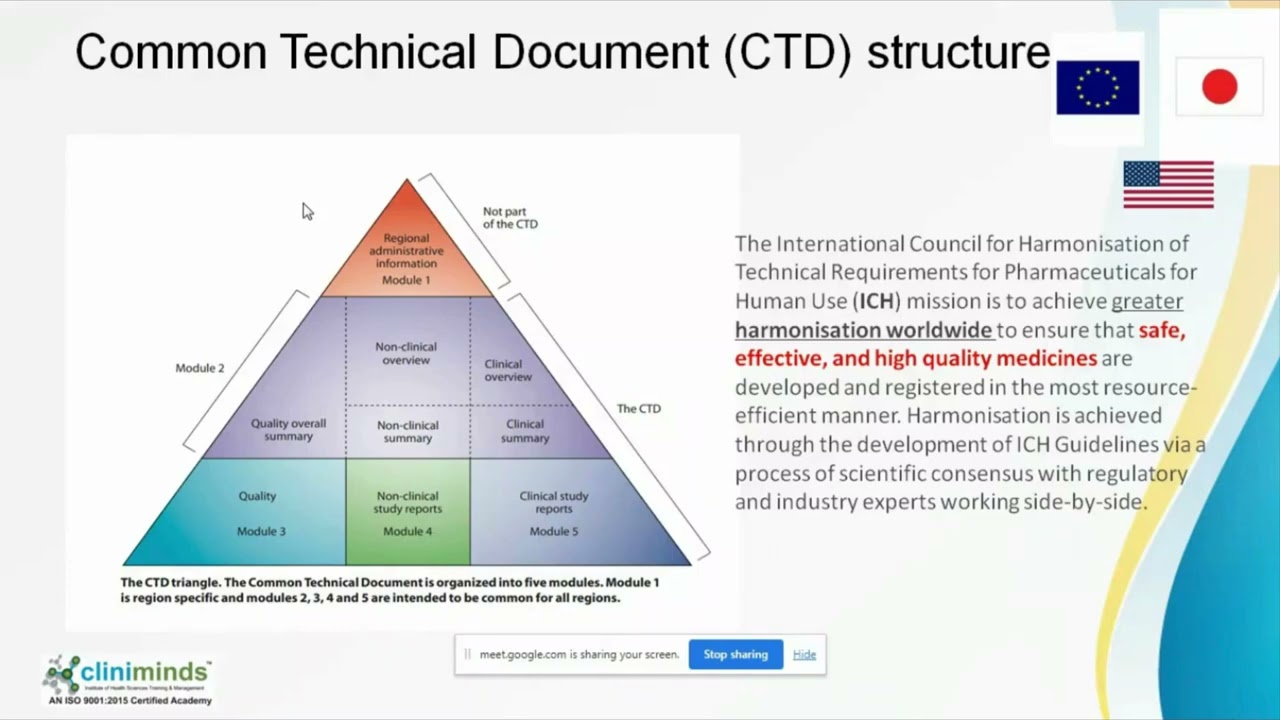
The Common Technical Document (CTD) is a standardized format for submitting drug approval applications to regulatory authorities worldwide. It includes five modules covering administrative, quality, nonclinical, and clinical information. Developed by ICH, it streamlines global submissions and regulatory reviews.
#CommonTechnicalDocument #CTDformat #CTDsubmission #ICHGuidelines #PharmaRegulatory #DrugApprovalProcess #RegulatoryAffairs #ClinicalDocumentation #eCTD #PharmaceuticalIndustry #PharmaStudents #RegulatoryProfessionals #ClinicalResearch #MeDrugRegulatoryAffairs #regulatoryaffairs #Pharmacygraduate #Qualitycontrol #qualityassurance #qa #qc #quality
Повторяем попытку...
Доступные форматы для скачивания:
Скачать видео
-
Информация по загрузке:



















