PCM and Regulatory It’s All About the Data
Автор: Pharmatech Associates, a USP Company
Загружено: 2026-01-05
Просмотров: 15
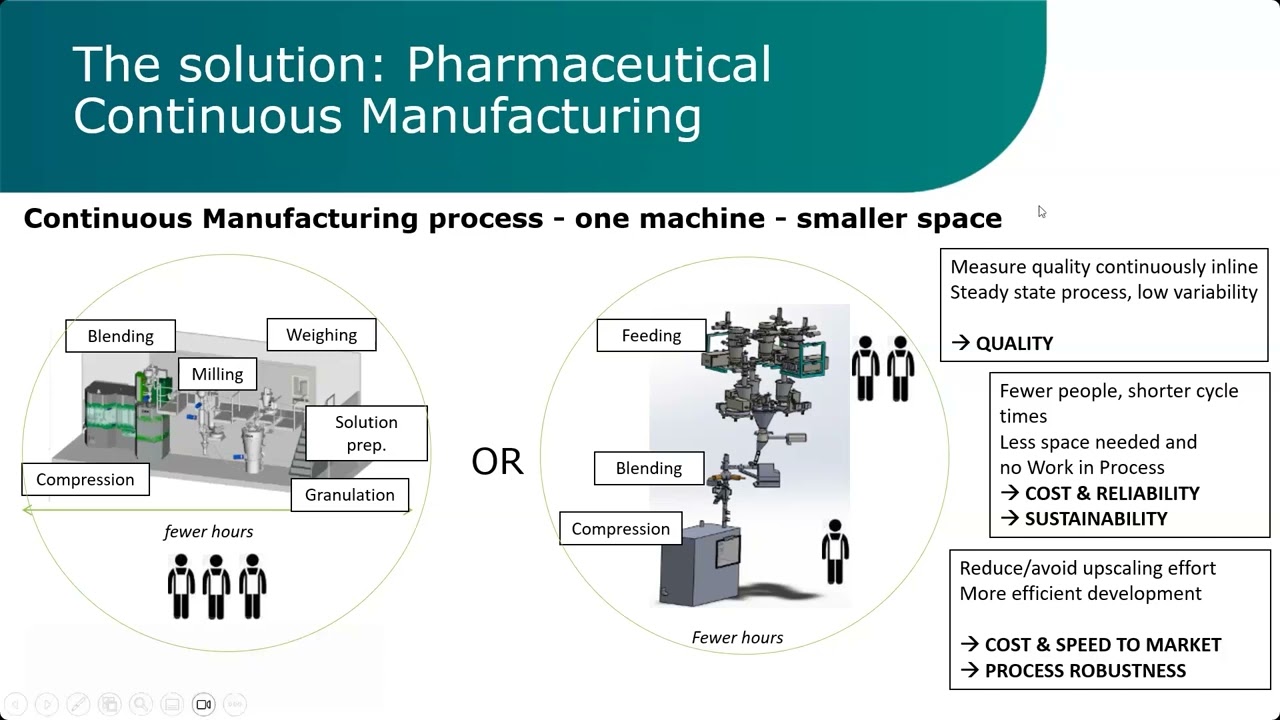
Описание: This webinar will guide you through the expectations of regulators when filing a Pharmaceutical Continuous Manufacturing (PCM) process for an Oral Solid Dose (OSD) drug product. Starting from the difference between a batch and a continuous process we cover the impact of evidence that needs to be generated so that the quality of the released product is assured, and how this will evolve throughout the drug product lifecycle. FDA, EMA, ICH [Q13].
Повторяем попытку...
Доступные форматы для скачивания:
Скачать видео
-
Информация по загрузке:



















