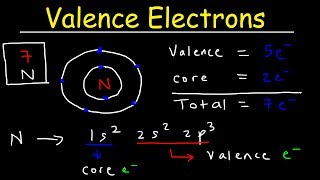
Identifying Elements with Valence Electrons in the s-Orbital – WAEC 2023 Chemistry Q38
Автор: David Soughtout
Загружено: 2025-11-18
Просмотров: 190
Описание:
✅ LONG CAPTION (LinkedIn & YouTube Shorts)
WAEC 2023 Chemistry Question 38
Which of the following elements has its valence electrons in the s-orbital?
Options:
A. Aluminium
B. Phosphorus
C. Sodium
D. Carbon
Aluminium (Al): 3s² 3p¹ → valence in p-orbital ❌
Phosphorus (P): 3s² 3p³ → valence in p-orbital ❌
Sodium (Na): 3s¹ → valence in s-orbital ✅
Carbon (C): 2s² 2p² → valence in p-orbital ❌
Clearly: Only Sodium has its valence electron in the s-orbital.
👉 Correct Answer: C — Sodium
💡 Exam Tip: Group I metals always end with ns¹ → valence electron in the s-orbital.
#waec2023 #waecchemistry #electronconfiguration #sorbital #studywithme #chemistrystudents #exampractice #jambprep #necoprep #chemistrytips #nigerianteachers
waec 2023 chemistry
s orbital valence electrons
electron configuration tutorial
waec chemistry past questions
chemistry basics nigeria
periodic table electron shells
Повторяем попытку...

Доступные форматы для скачивания:
Скачать видео
-
Информация по загрузке: